SPIM
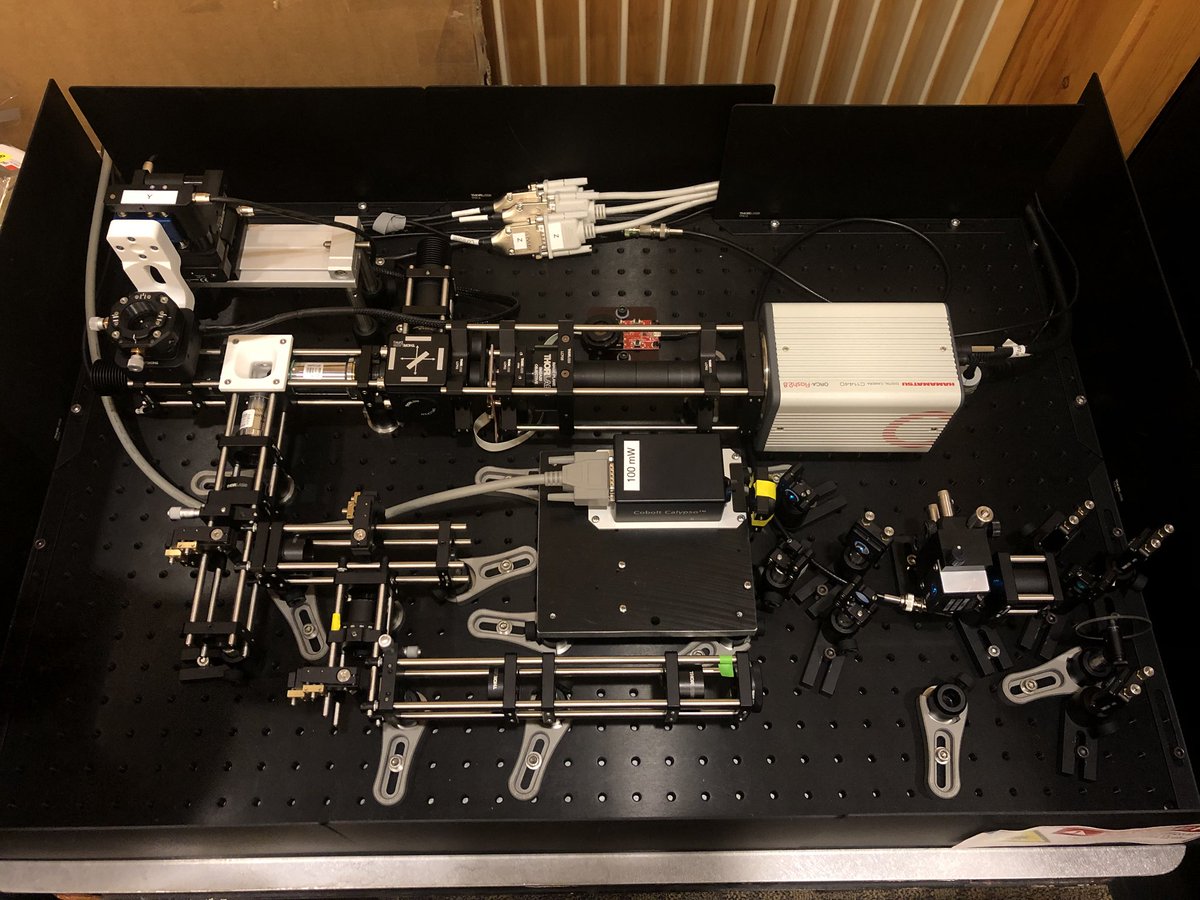
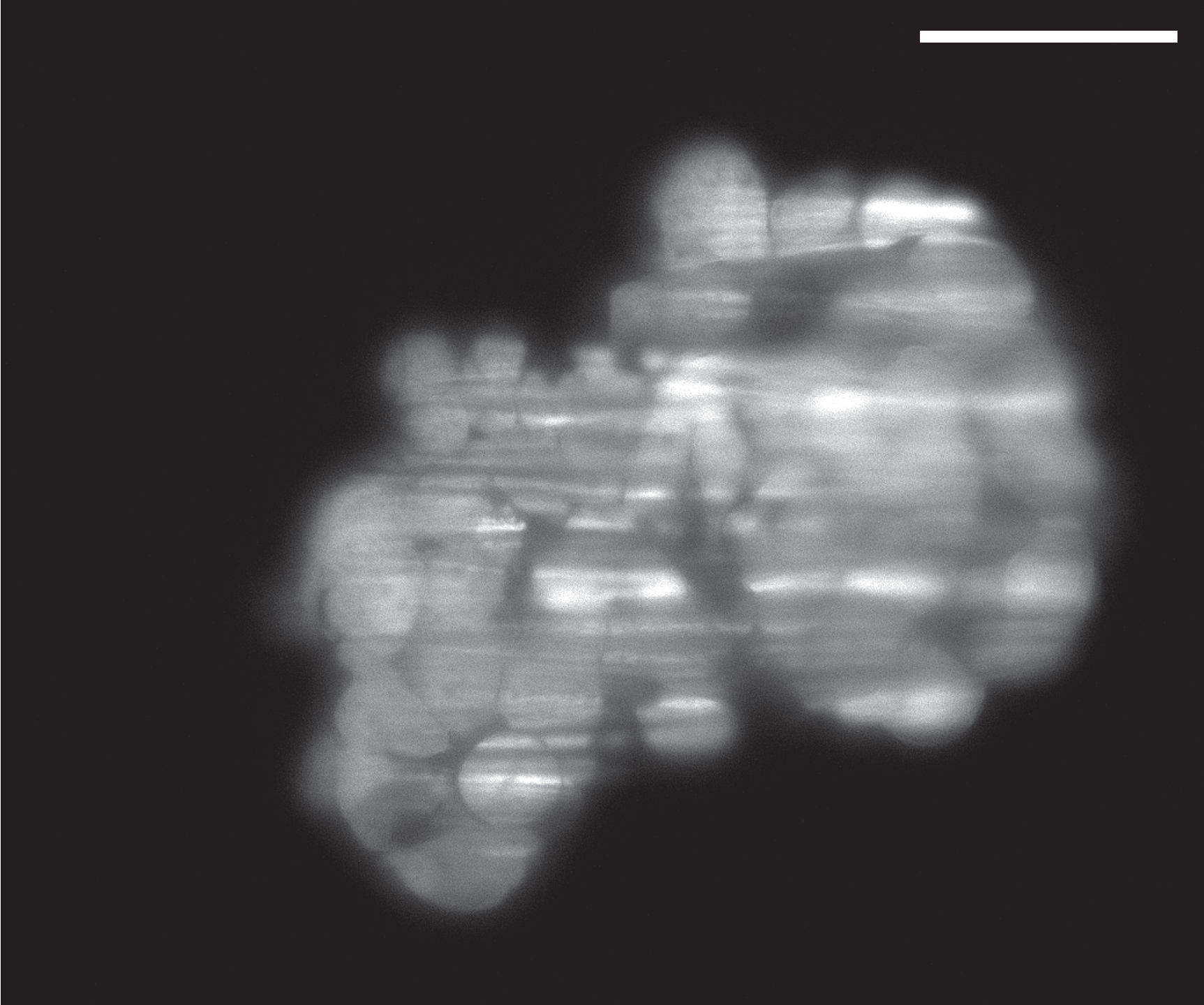
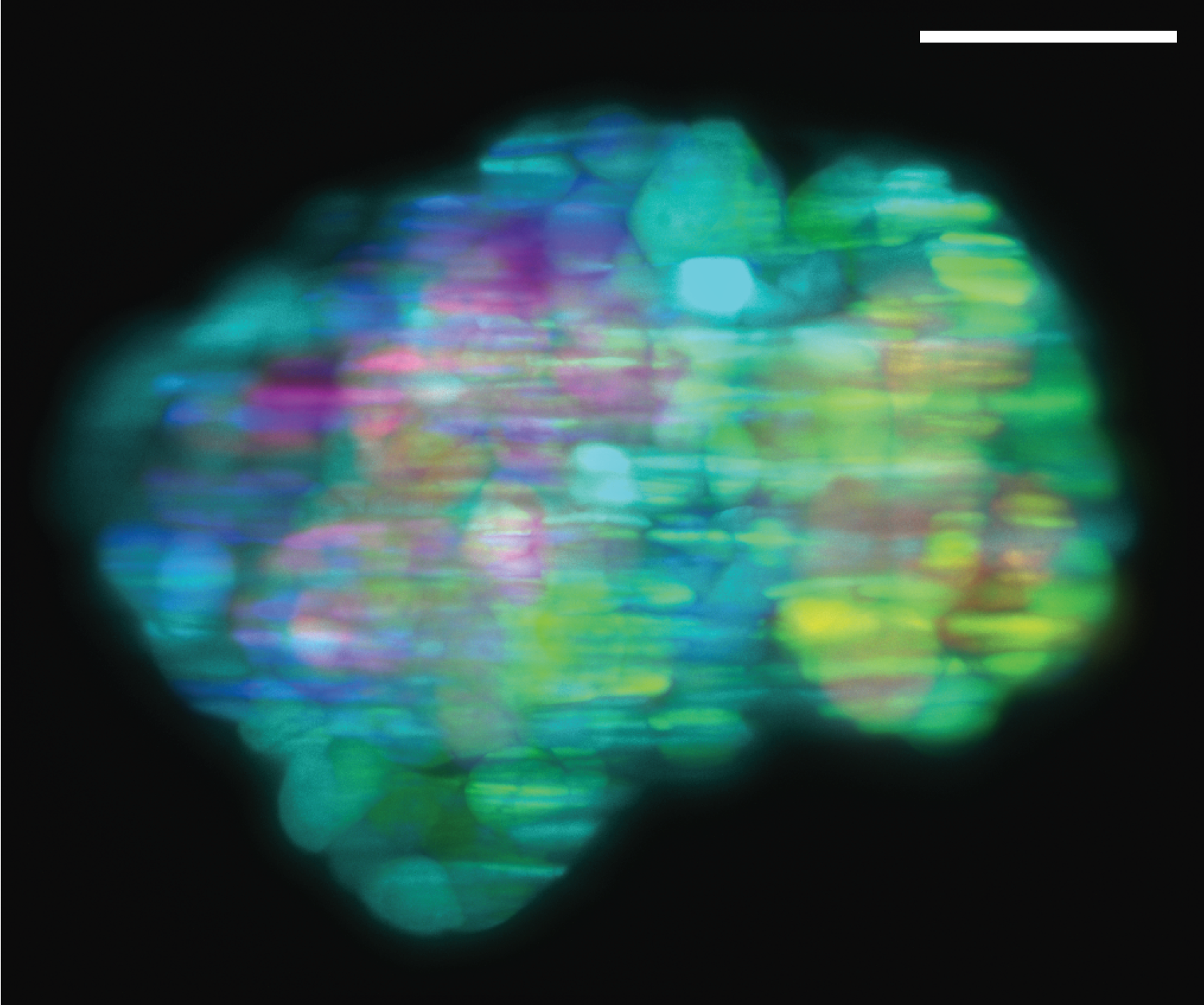
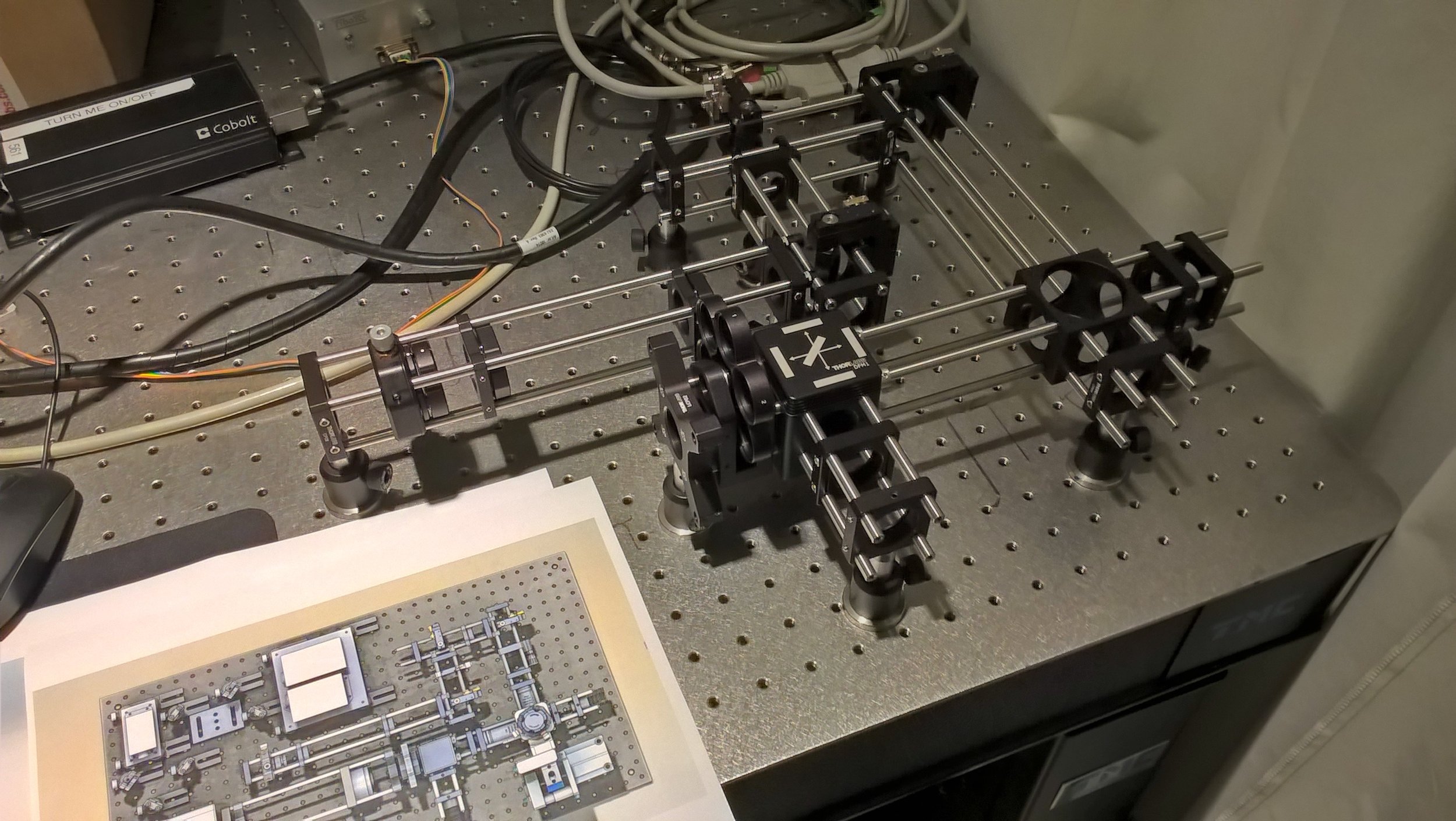
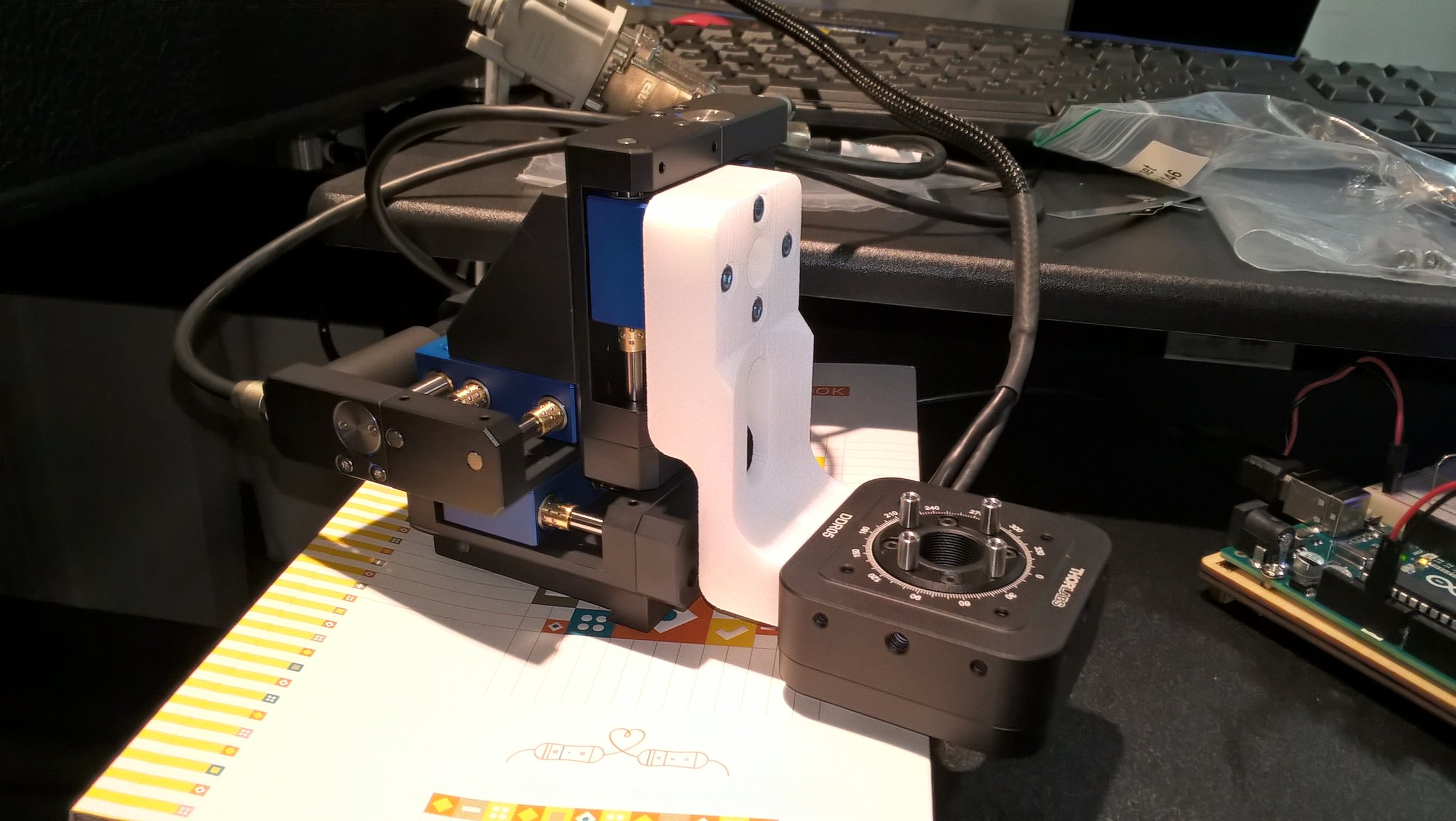
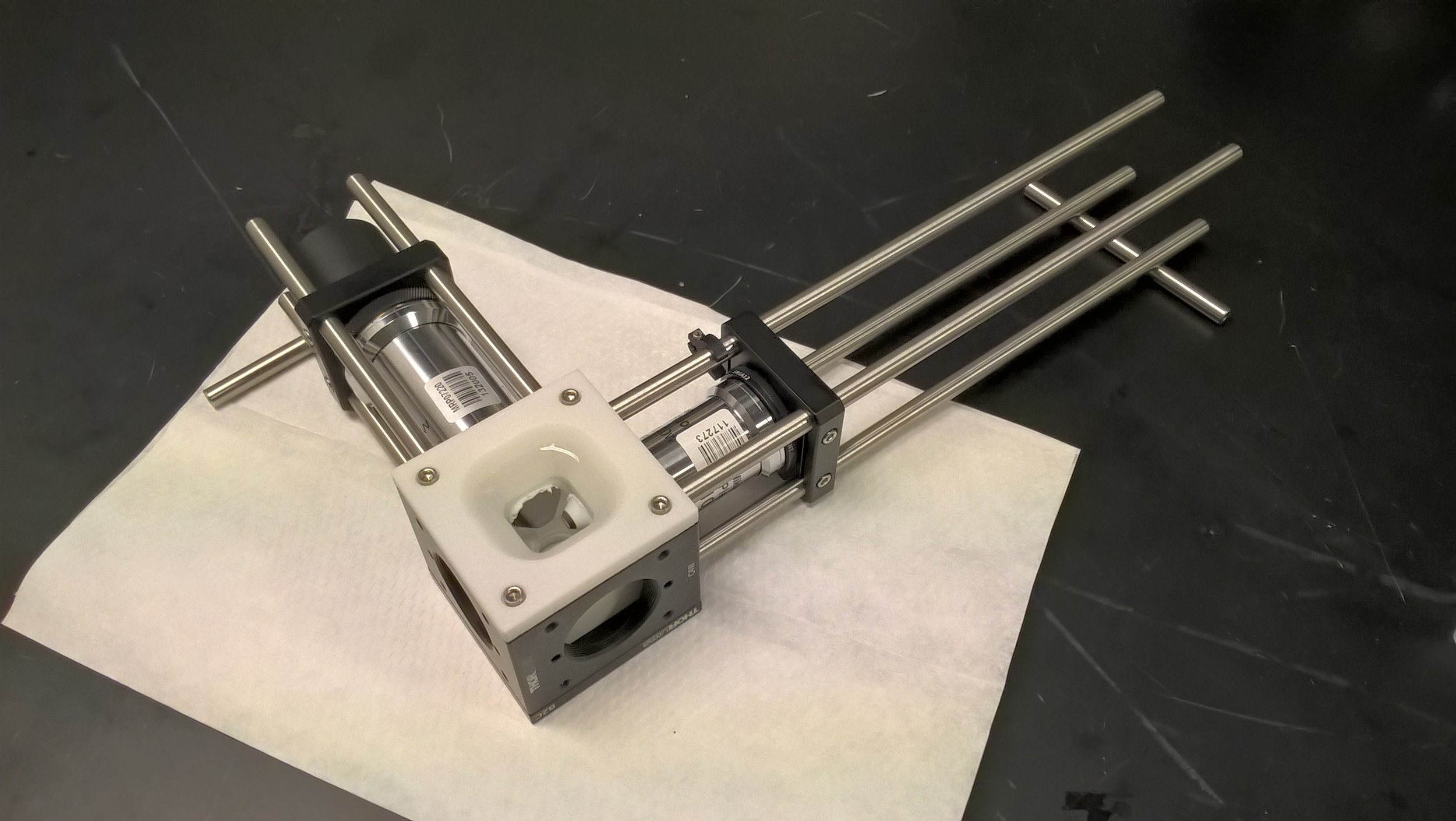
Selective Plane Illumination Microscope (SPIM) for research and education. Combining concepts of the OpenSPIM and of advanced home-built light sheet microscopes. Optimized for classic light sheet microscopy of samples like Drosophila embryos and spheroids, while maintaining a small footprint for mobility and a reasonable price point. Single-sided illumination with a static Gaussian light sheet. Integrated epi-fluorescence and brightfield illumination, 16x/0.8 W objective. Widefield detection arm with an sCMOS camera. Motorized sample rotation & translation. Most of the parts are off-the-shelf, with only a handful of custom components. The system is controlled by LabVIEW (Base Edition). My ELMI 2017 poster summarizing the microscope setup is available on figshare.


![Spim_solidworks-screenshot09[1].png](https://images.squarespace-cdn.com/content/v1/5b154b5389c172f985b03f38/1537454542742-LFOYDD9J48PCX1IWXA2N/Spim_solidworks-screenshot09%5B1%5D.png)